Abstract
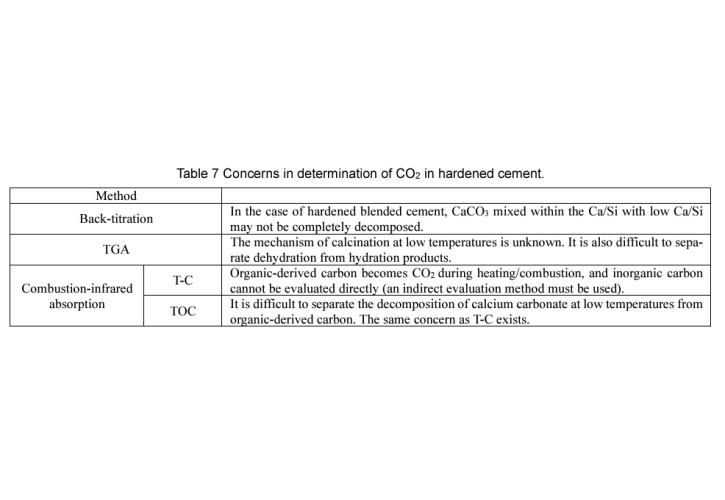
In the cement and concrete industries, technologies are being developed to reduce CO2 emissions by fixing it as inorganic carbonates within cementitious materials. This study quantitatively analyzed the CO2 content in cement pastes under different carbonation conditions using back-titration, thermogravimetry (TGA), and combustion-infrared absorption methods. The analysis of silica gel produced from the decomposition of C-S-H and gas components generated during the thermal analysis of carbonated samples allowed for an evaluation of the sample properties and an examination of the errors associated with each quantification method. Back-titration effectively decomposed carbonates and cement hydrates, accurately quantifying CO2 regardless of the composition and amount of silica gel. In contrast, TGA tended to underestimate CO2 as it failed to detect CO2 derived from calcium carbonate polymorphs at lower temperatures. The combustion-infrared absorption method showed slightly higher CO2 quantification compared to back-titration due to the influence of residual organic matter, and equipment without a desulfurization tube tended to quantify even more CO2.
H. Takahashi, I. Maruyama, Quantification of CO2 in cement pastes with different degrees of carbonation, Journal of Advanced Concrete Technology 22 (2024) 706–715. https://doi.org/10.3151/jact.22.706.
https://www.jstage.jst.go.jp/article/jact/22/11/22_706/_article/-char/en